She has managed to settle a protracted scholarly dispute that had been dragging on since the 90s, solving one of the puzzles of how genetic information is copied. Her undergraduate thesis was awarded the ‘Junior Nobel Prize’. Now, after her PhD and post-doc studies in Denmark, she has come back to the Czech Republic, establishing her own lab and team. The molecular biologist Hana Polášek-Sedláčková.
Read the story in Czech translation here
Every second, our bodies are producing approximately 3.8 million new cells – most of these are blood cells, others digestive tract cells, and a mere 2% account for the rest of the body. Neither the body’s regular maintenance, nor the genesis of a new human being would be possible without a key process that takes place in the very cell nucleus: DNA replication. It allows genetic material to be copied from one cell to another. For molecular biologist Hana Polášek-Sedláčková, the process is becoming ever more fascinating.
“It’s so wonderfully precise! If it weren’t for the translation of genetic information from one cell to another, and from generation to generation, none of us would be here,” says the thirty-one-year-old scientist, who has a long track record of success: from winning the Young Czech Brains (České hlavičky) competition for gifted youngsters, through a gold medal in a global undergraduate thesis contest, to publications in Nature and Science.
Her journey shows that supporting enthusiasm and talent, starting at primary school, can yield world-class scientific results. In Hana Polášek-Sedláčková it also combines with the patience and perfectionism inscribed into her DNA from both her grandmothers – one was known around the family for giving everything 120%, and the other showed her granddaughter exceptional tenacity by spending evenings embroidering traditional motifs on folk costumes, stitch by stitch.
Just please don’t blow up the house
Hana Polášek-Sedláčková grew up in rural South Moravia – in Svatobořice-Mistřín, a village near the town of Hodonín. Her passion for biology was awakened early, by a teacher who ran a primary school science club. Hana took part in various science contests and correspondence assignments, about the environment, for instance. Indeed, initially, she thought she would become an environmental scientist. She got involved in a nationwide initiative called the Czech Children’s Assembly for the Environment (Sněm dětí ČR pro životní prostředí) in which, over the course of four years, schoolchildren carried out various assignments on a different topic each year: water, the atmosphere, fire, soil…
“At that time, I had a lab in my parents’ basement, which was constantly changing, depending on what I was dabbling in at the time. At one point it was fossils, then plants I collected around a local pond, one year I was looking into energy, measuring power consumption on model fridges, freezers, and hotplates,” she reminisces. “The only reservation my parents had was about where my lab was located – they’d say that if one of my experiments ended up exploding, it would blow up the whole house,” the scientist laughs.
Her parents always supported her education, and that of her two elder siblings, even though – or perhaps for that very reason – they themselves do not have university degrees. “My mum works in a hospital, organising ambulance dispatches, and my dad works in a screw factory in Kyjov. They’ve always wanted us to get degrees and make it further than they had the chance to,” she explains.
As for her, she has always simply followed whatever she enjoys. Ironically, she disliked secondary school biology lessons, where she says the students were constantly reminded they were lagging behind the curriculum and that their main task was to catch up. In contrast, she was interested in chemistry. And she always longed to do ‘a little extra work’.
She was fond of biology contests and field trips, yet over time they began to seem too removed from what she wanted to pursue; observing sedge under a magnifying glass to identify different species by the presence and placement of trichomes (‘hairs’) on one side or the other was not what she was after.
It may have been in her first year at grammar school, however, during a two-week study retreat with biologists, that she happened to attend a lecture in which she first heard about the so-called central dogma of molecular biology: the journey of genetic information from the familiar double helix of DNA, through its replication in RNA, to the transcription of the original information via RNA into particular proteins and their behaviour.
“I was absolutely thrilled by the possibility to trace it down in such detail. All the way to the cell nucleus! And to observe the processes through which genetic information is stored,” she recalls.
Then her homeroom teacher, Stanislav Chuchro, made a subtle intervention – at just the right moment, he pinned a flyer on the classroom bulletin board, advertising university research internships for secondary school students, as a part of a so-called Secondary School Vocational Activity (Středoškolská odborná činnost) programme. “He’d always say he didn’t really care about where we were headed, though I think he knew how to point us in a certain direction and make it seem like we figured it out on our own. He was always encouraging us to be independent. But we had his support, too,” Hana Polášek-Sedláčková says.
So, shortly before turning eighteen, she applied for an internship and set off for the labs on the campus of Masaryk University in Brno, which was then still under construction in the Bohunice district. “I was completely clueless about where I was going. I headed off to the Faculty of Sports Studies. There they had no idea what it was I was looking for, so I ended up haphazardly asking passers-by, calling my family…,” she describes those tense moments. After an hour of walking in circles, she eventually arrived at the lab of biochemist Lumír Krejčí. This marked the start of a long and inspiring collaboration.
“I often find myself in situations in which little things make a lot of difference. If things hadn’t panned out and Lumír Krejčí hadn’t waited that extra hour for me, my career may have followed an entirely different route,” she reflects.
I’ll give it my best shot and we’ll see…
Thus, while still at secondary school, she gained access to a real lab, which was vastly different from the one in her parents’ basement. “I felt like Alice in Wonderland,” she recalls. In Lumír Krejčí’s lab, the main focus was DNA repair – radiation, chemicals, or cell metabolism can cause as many as tens of thousands of defects in a single cell every day, yet cells are capable of repairing such DNA damage. And it is proteins that play a key part in the process.

“I remember my first talk with Lumír about how research works. He told me it was a lot about results, about publications. And that if you’re interested in a topic and put your nose to the grindstone, you can ultimately pick and choose what lab you head for,” says Hana Polášek-Sedláčková.
It was then that she made up her mind to try and give it her best shot. “And if it doesn’t come off, then I’ll quit research,” she told herself then, as she has on a few other occasions during her career. But it did come off.
Her secondary school provided Hana Polášek-Sedláčková with an individual learning plan: one year, for instance, she alternated lab weeks and school weeks, catching up on tests and exams later. And she would also spend virtually all her holidays in the Brno lab.
And she carried on in much the same way after she enrolled at Masaryk University. At last, the university system gave her the freedom to dedicate maximum time to what she really enjoyed. “I didn’t feel like sitting through lectures, I preferred staying in the lab and then just cramming for the exams,” she says.
She became preoccupied with then under-explored RECQ4 protein, helping to describe and understand it through gradual biochemical experiments.
RECQ4 belongs to a group of proteins that she nicknames ‘little angels’ – DNA guardians that, when damage occurs, summon ‘worker’ proteins to fix the defect. If RECQ4 fails to work properly, its mutation causes a rare hereditary condition called Rothmund-Thomson syndrome, whose symptoms include a red rash and cataracts.
Why did she study this particular protein? At the very beginning, Lumír Krejčí offered her several potentially interesting research topics to choose from. “I was attracted to this protein due to the link between the mutation and the disease, and also because the project was in its early stages. I took it up expecting to learn more methods, especially molecular cloning. That’s probably what interested me the most at that point,” she explains.
And so she employed biochemical approaches to observe the properties of ‘her’ protein in test tubes. “We were working with so-called purified proteins – by extracting the protein from cells you get it in ‘pure form’ in a test tube and then you can add whatever you like to it,” she elaborates.
Such additions might be other proteins, or differently structured DNA – although the double-helix form is the most familiar, various cell processes also result in other DNA shapes, such as forks and bubbles. Hana Polášek-Sedláčková researched what DNA shapes this protein bonds with most frequently.
Through experiments, she and her fellow researchers eventually discovered that the RECQ4 protein attaches itself to complex, cruciform DNA structures called Holliday junctions. Subsequently, it brings another protein to the spot, a repair protein that ‘splits’ the DNA structure, producing again the well-known double helix. By doing so, it helps avoid the accumulation of such structures in the cell, which could even lead to cancer.
The Junior Nobel Prize
She summed up all her findings from five years of experiments and research in her undergraduate thesis. And one evening, while she was checking her inbox before leaving the lab, she received a shock. She was informed that her thesis had been shortlisted for the Undergraduate Awards, a worldwide contest also labelled the Junior Nobel Prize. She now found herself competing with 27 of the best students from all around the world – from universities like Harvard and Yale – for a gold medal in the Life Sciences category. In total, over 5,000 students from 39 countries submitted their undergraduate thesis to the competition.
Hana won. To her own amazement. At an awards ceremony in Dublin, the President of Ireland presented her with a gold medal for the overall winner in her category. The jury commended the fact that her thesis was a full-fledged, comprehensive study rather than just a small-scale project. After all, over the five years in the lab, she had made hundreds of measurements and contributed entirely new insights about a little-known protein.
“It was a terrific experience. It made me fully realise that research was happening all over the world and that we were all competing with one another. I’d thought that to compete with the world’s top universities was beyond the bounds for someone from Brno. The award actually came to me as quite a shock,” she recalls now, seven years later.

As a postgraduate student, she published a paper on ‘her’ protein in DNA Repair, so that thanks to her meticulous work the world now knows what RECQ4 actually does.
Love through a microscope
As time went on, however, the young researcher began to gravitate towards other approaches. “I found biochemistry interesting, but rather abstract: we extract what we need from cells and then check in a test tube how the protein behaves. But things can work differently inside a cell, because a cell is a more complex entity,” she explains.
She saw this for herself towards the end of her undergraduate studies, when she travelled to Denmark for a brief internship at a lab run by Ian Hickson, whom she had met shortly before in Brno, where he had been invited by her supervisor Lumír Krejčí (Hickson and Krejčí were exploring similar topics).
It was only at Hickson’s lab that she saw actual cells under the microscope. And it was love at first sight. She says herself it was then that she fell head over heels for the study of basic cell processes, and for cell biology. “I still remember that day, and I think it was really my greatest experience. I was unable to tear myself away from the microscope.”
Those particular cells had been extracted from chickens and, in hindsight, Hana Polášek-Sedláčková concedes they did not adhere to the slide as well as other kinds, which made them hard to handle. “But looking into the microscope and seeing some parts, in the nucleus, for example, marked with fluorescent dye… That was absolutely marvellous,” she recounts enthusiastically.
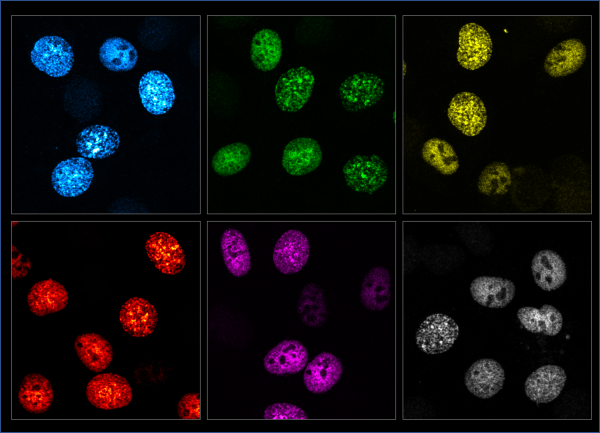
Blue, green, red, and magenta. With these dyes – fluorescent proteins extracted from jellyfish – scientists ‘turn the spotlight’ on whatever it is they need to study: like, for instance, parts of a cell nucleus. This simple but effective tool earned a trio of scientists the 2008 Nobel Prize in Chemistry.
Observing processes in the ‘lit-up’ cell in real time, first hand, was a turning point for the researcher, steering her course towards cell biology.
But where to go from there? During her postgraduate studies, after coming back from her Danish internship, she worked on the already mentioned paper and assisted her colleagues on their research projects. At the same time, she began to delve deeper into cell biology, looking for where to proceed. She was carving her own path. “Occasionally, I wasn’t quite sure whether I was using the fluorescent dyes correctly. And it seemed to me the best way would be to learn from leading experts in the field, who have microscopy down pat,” she says.
Her efforts to move on were encouraged even by her supervisor. “He knew how to be supportive. Unlike some supervisors, he was able to say he’d like a student to stay on, but that it’d mean no progress for them, research-wise. He was able to help his students in their careers without taking things personally,” the researcher appreciates Lumír Krejčí’s attitude, believing he adopted it in the US where he had worked.
Hana Polášek-Sedláčková had her sights set on several prospective places, like Oxford and Copenhagen. Eventually, she opted for the latter, one of her reasons being that the Danish research community is somewhat more open and responsive. “The Oxford people were more close-handed about their research topics, whereas in Denmark they had no qualms about sharing their insights and discoveries. Today, I’m in a position to say that having a talk with a fellow researcher can steer you towards uncharted waters, towards stones unturned. It can point you towards new, interesting ideas,” she believes.
Later, when Lumír Krejčí referred her to a particular contact in Copenhagen, she was surprised by the person’s name: Jiří Lukáš. “I thought: Oh! Sounds Czech, how did I miss that? So I did some digging and found out he was the number one authority on the cell cycle, genome stability, cell biology, and microscopy as such,” she explains.
Jiří Lukáš ranks among the most cited Czech scientists and has ‘struck gold’ on a number of occasions – a few times together with another science superstar, Jiří Bártek, who holds the very highest citation score among Czech researchers. They started out together at the Danish Cancer Society, studying, for instance, the cell cycle and how it is regulated.
They discovered that over its life cycle, a cell passes through different phases and milestones – or ‘checkpoints’ – that decide whether it should go on or not. “The discovery of these checkpoints is more or less their achievement. They described the molecular mechanism of how it works. So, that’s the two of them for you,” says Hana Polášek-Sedláčková.
The MCM paradox
So she wrote to Jiří Lukášek, asking whether he would have her as a PhD student. Although she had to pass through a standard admission procedure, it was apparent he was intrigued: Lukášek keeps his lab team small and is very selective about new recruits, yet he repeatedly asked her to confirm that she would indeed apply. She did, and she was admitted. “This proved what Lumír Krejčí had told me right at the start: taking an interest and putting in the effort will get you anywhere you want to be,” she observes.
Her work in Copenhagen had little in common with what she had been doing in Brno. She started to pursue the cell biology she had been longing for: working with cells, microscopes, and DNA replication, which she had already been attracted to during her years in Lumír Krejčí’s lab, but where she and her fellow researchers had mainly focused on DNA repair.
Her dedication to the topic of replication continues to this day. From the beginning, she was attracted to it not only for its being such an essential process that life would be impossible without it, but also for its being somewhat mysterious. “Few people in cell biology tackle the fundamental questions of DNA replication. The reason for this reticence is that some proteins which are absolutely essential for the process couldn’t be visualised in the cells,” she says.
She and her colleagues in Jiří Lukáš’s lab, however, managed to surmount this obstacle.
After her arrival, the very first thing she studied under the microscope were so-called MCM proteins. “Look at what we’ve learned from research papers,” Kumar Somyajit, her then tutor, pointed out. “And now look into the microscope. Not the same, is it?” “But how’s that possible?” Hana Polášek-Sedláčková wondered. “Nobody’s been able to tell. Not since the 90s, when these proteins were discovered. It’s even been named the MCM paradox,” Somyajit replied.
At world conferences, these proteins had been stirring up heated debates. Biochemists believed these substances played a key role in copying genetic information from one cell to another, i.e. in DNA replication. That they were so-called helicase proteins, which unravel the strands of DNA’s double helix so that it can be copied. Cell biologists objected that this was impossible. That it simply did not add up.
“That’s where the dispute stalled. For the next few years, cell biology slept on the issue and didn’t engage with it, because there was no way to visualise the process in the cell. When Kumar showed me the paradox under the microscope I did find it really odd, but I didn’t see it as my job to figure it out. I just thought that, given time, we’d perhaps learn more,” she says.
So she began to study the MCMBP protein – for a similar reason as when she investigated the RECQ4 protein back in Brno: a fellow researcher’s experiment flagged it as interesting, and little was known about it.
“It wasn’t easy because we had no tools to study the protein with. We had to develop the imaging methods from the ground up,” Hana Polášek-Sedláčková comments.

In this she was helped by Claudia Lukáš, the wife of the lab director and leading expert on microscopy. “She had a great nose for methods. Just getting a cue from her made it clear that the topic was worth your time and effort. She suggested that if I wanted to look into the protein’s dynamic, the best way would be to study it in live cells and tag it through CRISPR-Cas9 gene editing,” the researcher remembers.
Simply speaking, this means using ‘gene scissors’ to insert fluorescent dye wherever one needs it. Two years ago, this widely applicable method also earned its authors, Emmanuelle Charpentier and Jennifer Doudna, the Nobel Prize.
The method, however, had not yet been employed at Jiří Lukáš’s lab and so, again, it was up to Hana Polášek-Sedláčková to get the hang of it on her own. “Although I was intimidated at first, the experiments were moving along at lightning speed, because the lab was at a very high level. So, I blazed the trail and the whole lab is now following the protocol I set down,” says the biologist nonchalantly.
Although her journey may seem idyllic, she admits there were moments in her PhD studies when things were simply not going well. “We were testing different hypotheses and it wasn’t working out. On top of that, the weather was depressing in Denmark and the COVID pandemic broke out, closing up the borders, so it was a long time before I could see my boyfriend and family,” she recounts.
“I kept saying to myself I wasn’t going to lose my mind over it. That if it didn’t work out, I’d go and try something else. Like popularising science. Back at Masaryk I’d spent a while at the Bioskop Learning Centre and I enjoyed expanding the students’ education, mainly through experiments,” she suggests a possible escape route that she, however, never had to take.
“That’s what’s beautiful about science. You may do perhaps a dozen experiments that don’t come off. And the very next one yields something ground-breaking that defines all your work,” she reflects.
The Danish golden years
She dubs the time when she had the chance to do her research in the Danish lab the ‘golden years’. Her tutor, Kumar Somyajit, published – also with Hana Polášek-Sedláčková’s assistance – in the prestigious Science, and she herself had a paper accepted by the equally distinguished Nature.
That is where she managed to puzzle out the aforementioned MCM paradox that she had encountered at the very beginning of her time in Denmark. “And yet we hadn’t originally intended to answer this particular question. We were testing other hypotheses, but the results that were rolling in brought us to the paradox that’d been described many years before,” she comments on the wonders of doing basic research.
Thus, she gradually worked her way towards grasping what function the mysterious MCM protein actually fulfils in the cell. “What intrigued us was that a cell produces these proteins in huge quantities, but only a fraction is activated during replication. We found out that the process in the cell is actually much more complex: MCM exists in different forms – a parent and a newly formed one. It’s very likely that the parent proteins carry some information from the previous cell cycle into the daughter cell, because they are preferentially activated on replication forks (the sites on the double helix where DNA replication takes place). The new MCM proteins, whose quantity is double that of the parent proteins, stay mostly inactive. An elegant tool for fluorescent staining, the so-called HaloTag, which allows you to distinguish between the two MCM protein forms, led us back to the question of why there are so many MCM proteins in the cell in the first place,” the researcher describes her discoveries.
She and her colleagues learned that the new MCM proteins which remain largely inactive during DNA replication work as ‘natural inhibitors’ of the replication forks, so that replication does not proceed too rapidly and DNA can be copied without errors.
“What we learned is that the surplus inactive MCM proteins are quite important, even if they seemed useless at first. If they didn’t exist, the fork would be running too fast, racking up errors, which could end up causing genetic instability and the development of cancer,” says Hana Polášek-Sedláčková.
And what is more, they also discovered that these mechanisms are exploited by cancer cells. They intentionally produce these proteins in larger quantities to make sure they manage to copy their DNA and keep the tumour growing.
The research team even worked out which protein regulates how many of these inhibitors are used by the cell. It is, in fact, the MCMBP protein that was assigned to her at the very beginning. “We called it the ‘babysitter’.”
In their paper for Nature, they went on to describe how this could be a potential target for cancer treatment. If a chemical substance was produced to keep the ‘babysitter’ in check, cancer cells would have fewer inhibitors and therefore make more errors. If the errors added up, it would spell doom for the cancer cell.
Recently, she has also had a paper published in Nature Communications in which she reiterates how to ‘stain’ cells so that researchers can observe the DNA replication in question. She explains why it is that for such a long time, practically since the 1990s, scientists were unable to see the key protein in the cell.
Why then? “Actually, the solution is awfully simple. The problem is that those MCM proteins form the core of the replication fork and other proteins bond with them, obscuring them from view. This means they’re hidden, and the antibodies with fluorescent dye that are supposed to attach to them have no way of reaching them,” she explains.
The solution consists in the aforementioned CRISPR gene scissors. They allow the dye to bond directly with the protein creating a kind of ‘flag’ that gives away the protein’s location.
“When I showed Jiří Lukáš the first images of cells producing the CRISPR-flagged MCM proteins, he said to me right away: ‘Yes, that’s the problem. We’ve solved it.’ Solving something conclusively is a rare thing in science,” she remarks. The simplicity of her solution was praised even by the otherwise severe peer-reviewers of her paper.
“I get people asking me at conferences how come nobody figured this out earlier. What I explain to them is that in the 90s, proteins could only be visualised through antibodies. CRISPR didn’t appear until a few years later. Technology in science is pushing ahead and one shouldn’t be afraid to backtrack and try using up-to-date methods to re-examine initial hypotheses or the underlying scientific foundations.”
Things are changing in the Czech Republic, so I’ve come back
As Hana Polášek-Sedláčková was approaching the end of her four-year PhD study in Denmark, Jiří Lukáš proposed an extension – to continue with him as a post-doc fellow for as long as she wanted. So she ended up spending about six years in Denmark and had time to consider her next move.
She chose to return to the Czech Republic. “I was aware that they’d been spoiling us a little at the lab. We had a lot of freedom there and I didn’t want to lose that.” Her private life was also a factor. After several years of long-distance relationship, she and her husband decided they would settle in the Czech Republic. “What’s more, Czech research is moving forward and excellent funding opportunities are now opening here for up-and-coming researchers,” she explains.
The Czech Science Foundation provided her with a Junior Star grant from a new programme aimed at scientists within eight years of receiving their PhDs who have gained substantial international experience and published in prestigious journals.
Since February 2022, she has therefore been running her own team at the Institute of Biophysics of the Czech Academy of Sciences in the Brno district of Královo Pole. Why there? For one, she has a fondness for South Moravia and has noticed that Brno has a good reputation within the science community. And she was also tipped off about the institute by her one-time tutor Lumír Krejčí. “He recommended me a few people to contact. First of all, I wrote to Eva Bártová, the institute’s head. She responded promptly; right away, she was happy to support me and help me with grant applications,” says Hana Polášek-Sedláčková.
Moreover, until recently she was working directly at Eva Bártová’s lab, with full access to all its equipment, meaning she had no involuntary period of downtime – waiting for facilities and technology after her return from Denmark. Now she is moving to a different part of the same building, into her very own new lab.
Her current team comprises six people. They will be focusing on DNA replication or, more specifically, its fundamental, primary mechanisms. What in a cell triggers the concert that culminates in the transcription and copying of genetic information? “We’re looking into the first steps in replication and exploring new pathways involved in its regulation. If there are too many or too few proteins, or if they act at a wrong point of the cell cycle, it leads to genetic instability and the development of cancer. That’s our day-to-day topic. We study how the first step in DNA replication should work properly, so that it doesn’t end up with cancer,” she sums up.
A generous supervisor, Jiří Lukáš allowed his former PhD student to take off to her new lab with the special expertise she had carved out for herself under his guidance – her thematic niche, as he himself has reportedly called it. And that even included priceless cell lines and reagents that were flown to Hana, frozen, from Copenhagen.
The cell lines she created with her fellow researchers using the CRISPR molecular scissors are much in demand, so she has even started sending them to other labs around the world, as they are an important tool for scientists.
And she intends to continue working with the CRISPR-Cas9 in her lab: “It really is my favourite method: modifying proteins in the cell, dyeing them in different colours, and observing them under the microscope. Even though with this time-consuming method it may take as long as three months to see the actual results, it’s worth the wait,” she affirms.
It is lab work that she still finds to be the most fulfilling part of her job. “The sheer number of proteins that we can tag is huge. So there’s always more to discover. That’s really the bee’s knees,” she laughs.
Even nowadays, she is grateful to be distracted from her administrative duties by students wanting to show her intriguing results. “I always say: This is the interesting part – seeing results, exploring together, pondering and proposing new hypotheses and experiments. Not like paperwork,” she says.
There is no such thing as bad data or dumb questions
Apart from cell lines, what other aspects of her Danish experience does Hana Polášek-Sedláčková want to carry over to the Czech Republic? She, for instance, appreciated the outstanding ‘lab meetings’ – sessions with fellow researchers in which the best discussions would take place.
She has also learned not to be afraid and not to be put off if data does not turn out as hypothesised. “It’s sometimes the case in research that you stick to your original hypothesis and try to prop it up with data that doesn’t really fit. But that doesn’t really work. It’s better to keep an open mind,” she reflects, adding she has learned that “there’s no such thing as negative data, it’s just that we don’t yet understand what it’s saying”.
She would also like Czech scientists not to be afraid to occasionally come up with what might initially seem outlandish ideas or questions. “There’s this syndrome in the Czech Republic that students are afraid to ask a question because they’re shy, or because they think their question is dumb. There weren’t any such inhibitions in Denmark – things were simply discussed. One of the reasons may be that I never had a sense of hierarchical distinctions there: scientists would talk to one another as equals without any gesturing to superiority and inferiority you sometimes get in the Czech Republic,” the biologist comments on the situation.
What she also regards as beneficial is when, instead of paying attention just to their own topics, experts broaden their horizons and take part in interdisciplinary discussions. “In Denmark, scientists are interested in all sorts of things. They go to lectures on topics outside their field, because those distinct fields may suddenly come together,” she points out. She thinks the most interesting research and results emerge at these blind spots and disciplinary frontiers.
“What I’d like to emphasise is the importance of basic research. If it weren’t for basic research, we wouldn’t have the currently available cancer medications. Also, it was the extensive basic research of RNA and RNA vaccines that allowed the quick development of the COVID vaccine. In Denmark, the study of cell mechanisms and basic research were getting support, whereas in the Czech Republic the tendency is perhaps sometimes to slant support towards the application sphere. In short, both lines of research ought to be supported,” she opines.
PhD students are not just cheap labour
Another feature of Danish academia she would happily transplant to the Czech Republic is greater recognition for PhD students. “PhD students are the heart of a research lab and in Denmark they value them and have ways of generously rewarding their work with money. Since my return to the Czech Republic, I’ve been seeing that a lot has changed in Czech academia, but you can occasionally find institutions where PhD students are still treated as cheap labour,” she observes. “It’d be great if this changed, and their bosses came to appreciate students’ work and help them in their future careers. Just like I was helped by Lumír Krejčí,” she adds.
Money is an issue in its own right. The centre where she worked was funded by Novo Nordisk, a pharmaceutical company. “The very generous budget, however, had to be justified by outstanding results. Our centre was regularly subjected to very rigorous evaluation by a panel of international scientists, who then submitted their verdict to a foundation committee that conducted yet another round of evaluation according to its own criteria,” Hana Polášek-Sedláčková recounts the proceedings in Denmark.
In some Czech institutions, she says, the quantity of scientific outcomes still trumps their quality. “But if you have a look at the world’s best labs in the field of cell biology, you’ll see that they average one or two high-profile papers a year. Good scientists keep track of their community and if you churn out ten or twenty papers a year in minor, low-impact journals, what may happen is that other scientists won’t get to know your work and it will do nothing for the progress of worldwide scientific endeavour. We mustn’t be afraid of stepping out of the tiny Czech bubble and trying to do word-class research,” the scientist exhorts.
